Abstract
Background:
Diffuse large B cell lymphoma (DLBCL) is an aggressive malignancy of mature B cells. The disease has traditionally been subdivided into cell-of-origin (COO) subtypes - germinal centre B cell-like (GCB) or activated B cell-like (ABC) - as determined by expression profiling or immunohistochemistry of the tumor cells. However the role of the immune microenvironment, and how the tumor and immune system interact to influence patient outcomes, remains to be fully investigated.
Methods:
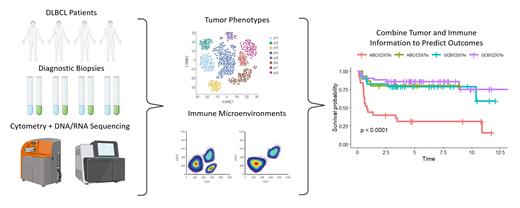
In this project, we used mass cytometry (CyTOF) to deeply profile both tumor cell phenotypes and the immune microenvironments, alongside ABC/GCB classification and mutation profiling, in a discovery cohort of 54 DLBCL cases. As well, a validation cohort of 129 DLBCL patients were immunologically profiled by high-dimensional conventional flow cytometry, and their immune profiles alongside ABC/GCB classification, mutation profiling, and RNAseq data, were correlated with patient outcomes as measured by progression-free survival (PFS).
Results:
Analysis of the CyTOF/discovery cohort demonstrated that DLBCL tumor cells are phenotypically unique to each patient, with a small number of samples displaying distinct sub-clonal structure, often distinguished by differential expression of immune-related proteins like MHC-II. ABC/GCB classifications could be recapitulated based on tumor cell phenotypes, demonstrating that while COO was a robust feature, a great deal of heterogeneity exists within these established subtypes.
Immunological profiling of the CyTOF/discovery cohort revealed that DLBCL samples could be divided into three distinct groups which roughly correlated with abundances of naïve, activated, or terminally differentiated T cells, respectively. This profiling schema was extended to the validation cohort of 129 patients which in turn led to identification of a subset of patients with a very high risk of disease progression (5-year PFS; 30% high risk vs. 80% low risk, p<0.0001).
This final classifier was based on a combination of ABC-DLBCL designation, combined with the presence of an immune microenvironment dominated by terminally differentiated (CD57+) T cells. We performed a limited series of functional studies using primary DLBCL biopsy samples to characterize further these CD57+ T cells as clonally restricted and incapable of responding to antigenic challenge. Interestingly, traditional immune markers of T cell exhaustion such as PD-1, TIM3, LAG3 and TIGIT were not correlated with patient outcomes.
Conclusions:
Overall, this study demonstrates the utility of immune profiling in risk stratification based on initial diagnostic biopsy material and highlights a subset of DLBCL patients who may benefit from immune-based therapies to rejuvenate the anti-tumor T cell response. We conclude that T cell senescence, rather than exhaustion, is the more relevant feature in DLBCL disease biology and highlights an alternate target for immunomodulatory therapy.
Craig: Bayer: Consultancy. Slack: Seagen: Consultancy, Honoraria. Scott: Abbvie: Consultancy; AstraZeneca: Consultancy; Celgene: Consultancy; NanoString Technologies: Patents & Royalties: Patent describing measuring the proliferation signature in MCL using gene expression profiling.; BC Cancer: Patents & Royalties: Patent describing assigning DLBCL COO by gene expression profiling--licensed to NanoString Technologies. Patent describing measuring the proliferation signature in MCL using gene expression profiling. ; Rich/Genentech: Research Funding; Janssen: Consultancy, Research Funding; Incyte: Consultancy. Steidl: Epizyme: Research Funding; Bayer: Consultancy; Curis Inc.: Consultancy; Seattle Genetics: Consultancy; AbbVie: Consultancy; Trillium Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding.